In an era of remarkable medical advancements, humanity faces a growing health security threat that could undermine decades of progress: antimicrobial resistance (AMR). Often called a “silent pandemic,” AMR occurs when bacteria, viruses, fungi, and parasites evolve to defeat the drugs designed to kill them. This comprehensive guide explores the causes, consequences, and potential solutions to one of the most pressing public health challenges of our time.
What Is Antimicrobial Resistance? Understanding the Basics

Antimicrobial resistance occurs when microorganisms (bacteria, viruses, fungi, and parasites) change over time and no longer respond to medicines, making infections harder or impossible to treat. While this natural evolutionary process happens gradually, human activities have dramatically accelerated it.
Antibiotic resistance—specifically concerning bacteria—represents the most urgent threat within the broader AMR challenge. According to the World Health Organization (WHO), antibiotic-resistant bacteria could kill 10 million people annually by 2050 if current trends continue, surpassing cancer as a leading cause of death worldwide.
At its core, AMR represents a Darwinian process: when exposed to antimicrobial drugs, susceptible microbes die while naturally resistant variants survive and reproduce. Over time, resistant strains become dominant, rendering previously effective treatments useless.
The Historical Origin and Evolution of Antimicrobial Resistance
Antimicrobial resistance is not a new phenomenon, but rather an accelerating crisis with deep historical roots:
The Dawn of Antibiotics
- 1928: Alexander Fleming discovers penicillin, marking the beginning of the modern antibiotic era
- 1940s: Mass production of penicillin begins, saving countless lives during World War II
- 1945: In his Nobel Prize acceptance speech, Fleming warns of potential resistance if penicillin is misused
The First Signs of Crisis
- 1950s-1960s: First major outbreaks of penicillin-resistant Staphylococcus aureus in hospitals
- 1962: Methicillin introduced to combat penicillin resistance; methicillin-resistant Staphylococcus aureus (MRSA) emerges within a year
- 1970s-1980s: Development of new antibiotic classes slows dramatically while resistance continues to spread
The Modern AMR Crisis
- 1990s-2000s: Emergence of multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis
- 2009: Discovery of NDM-1 (New Delhi Metallo-beta-lactamase), a gene enabling resistance to almost all antibiotics
- 2015: Discovery of mcr-1 gene conferring resistance to colistin, considered an antibiotic of last resort
- 2016: United Nations General Assembly holds first high-level meeting on antimicrobial resistance
- 2019: CDC’s report identifies more than 2.8 million antibiotic-resistant infections annually in the US alone
This historical trajectory reveals a concerning pattern: shortly after each new antimicrobial agent is introduced, resistance emerges. The “discovery void” of recent decades—with few new antibiotics reaching the market—has only intensified the crisis.
The Primary Causes of Antimicrobial Resistance
Multiple interconnected factors drive the acceleration of antimicrobial resistance:
Misuse and Overuse in Humans
- Unnecessary prescriptions: Up to 50% of all antibiotics prescribed are not needed or are not optimally prescribed
- Self-medication: In many countries, antibiotics are available without prescription
- Incomplete treatments: Patients not finishing prescribed courses of antibiotics
- Inappropriate use: Using antibiotics for viral infections like colds and flu, against which they are ineffective
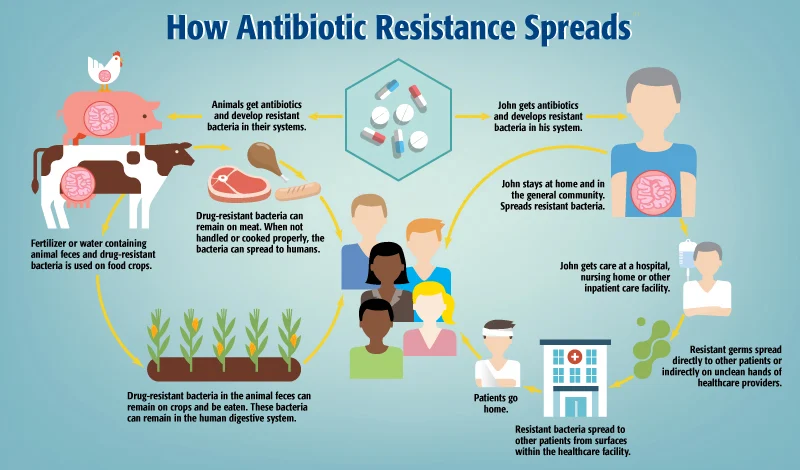
Agricultural and Livestock Practices
- Growth promotion: Low-dose antibiotics used to promote faster growth in livestock
- Disease prevention: Mass medication of healthy animals to prevent infections
- Similar drugs: Many antibiotics used in animals are identical or similar to those used in humans
- Environmental spread: Animal waste containing resistant bacteria and unmetabolized antibiotics contaminating soil and water
Healthcare Setting Issues
- Poor infection control: Inadequate hygiene and sanitization facilitating the spread of resistant pathogens
- Insufficient diagnostics: Limited rapid testing leading to empirical (best-guess) treatment
- Lack of stewardship: Inadequate programs to ensure appropriate antibiotic use
Pharmaceutical Market Failures
- Lack of new development: Limited profitability compared to drugs for chronic conditions
- Regulatory hurdles: Complex approval processes increasing development costs
- Conservation paradox: New antibiotics are typically reserved for last-resort use, limiting sales
The Major Health Threats: Priority Antibiotic-Resistant Pathogens
The WHO has identified the following “priority pathogens” that pose the greatest threat to human health:
Critical Priority
- Acinetobacter baumannii (carbapenem-resistant)
- Pseudomonas aeruginosa (carbapenem-resistant)
- Enterobacteriaceae including E. coli and Klebsiella pneumoniae (carbapenem-resistant, ESBL-producing)
High Priority
- Enterococcus faecium (vancomycin-resistant)
- Staphylococcus aureus (methicillin-resistant, vancomycin-intermediate and resistant)
- Helicobacter pylori (clarithromycin-resistant)
- Campylobacter (fluoroquinolone-resistant)
- Salmonella spp. (fluoroquinolone-resistant)
- Neisseria gonorrhoeae (cephalosporin-resistant, fluoroquinolone-resistant)
Medium Priority
- Streptococcus pneumoniae (penicillin-non-susceptible)
- Haemophilus influenzae (ampicillin-resistant)
- Shigella spp. (fluoroquinolone-resistant)
These pathogens cause common infections including urinary tract infections, pneumonia, bloodstream infections, and foodborne illnesses that are becoming increasingly difficult to treat.
The Consequences: Health and Economic Impact of Antimicrobial Resistance
The repercussions of antimicrobial resistance extend far beyond individual patients:
Health Impacts
- Increased mortality: Currently, at least 700,000 people die annually due to drug-resistant infections
- Treatment complications: Procedures like chemotherapy, organ transplantation, and surgeries depend on effective antibiotics
- Longer hospital stays: Resistant infections typically require extended treatment
- Return of untreatable diseases: Conditions once easily cured becoming life-threatening again
Economic Burden
- Healthcare costs: Extended hospitalizations and more expensive medications
- Productivity losses: Longer illness duration and increased mortality among working-age adults
- Global economic impact: Estimated cumulative cost of $100 trillion by 2050 if current trends continue
- Disproportionate effect on low- and middle-income countries: More limited access to quality healthcare and alternative antibiotics
Social Consequences
- Widening health inequities: Disparate impact on vulnerable populations
- Travel and trade restrictions: Potential international responses to contain resistant strains
- Food insecurity: Reduced livestock productivity and increased production costs
Warning Signs: Recognizing Antibiotic-Resistant Infections
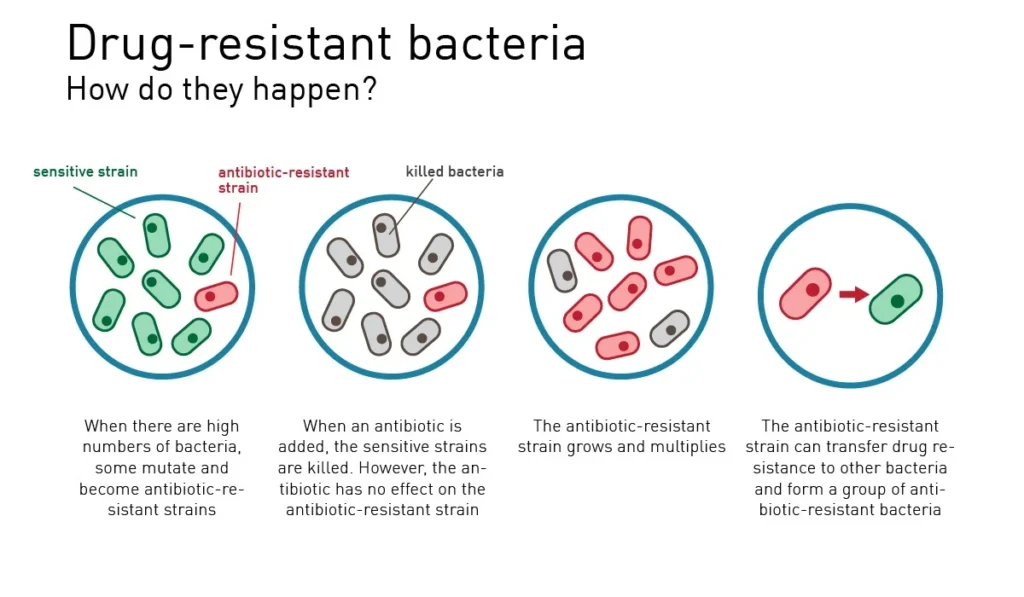
While laboratory testing is required for definitive diagnosis, certain warning signs may indicate an antibiotic-resistant infection:
- Symptoms that persist despite appropriate antibiotic treatment
- Recurrent infections that quickly return after initial improvement
- Infections acquired in healthcare settings, particularly in hospitals with known resistance problems
- Infections that develop after long courses of antibiotics
- Infections that fail to respond to multiple courses of different antibiotics
If you suspect an antibiotic-resistant infection, it’s crucial to communicate your concerns with healthcare providers and share your antibiotic history.
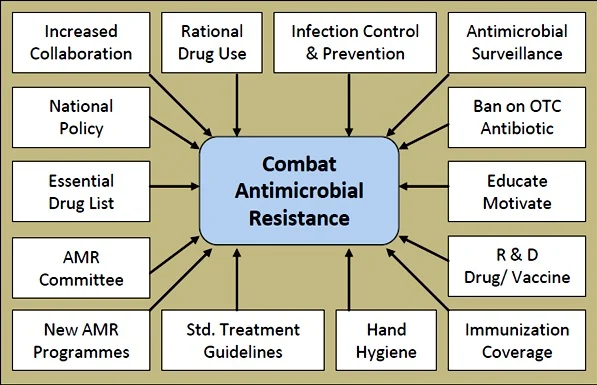
Global Strategy: The Multifaceted Approach to Combat AMR
Addressing antimicrobial resistance requires coordinated action across multiple sectors:
Policy and Governance
- National action plans: Over 100 countries have developed specific strategies to combat AMR
- Global surveillance: WHO’s Global Antimicrobial Resistance Surveillance System (GLASS) monitors and shares resistance data
- Regulatory frameworks: Policies restricting over-the-counter antibiotic sales and limiting agricultural use
- International cooperation: The Tripartite collaboration between WHO, FAO, and OIE coordinates global response
Healthcare Interventions
- Antimicrobial stewardship programs: Hospital and community initiatives to optimize appropriate use
- Improved diagnostics: Rapid tests to identify infectious agents and resistance patterns
- Infection prevention and control: Enhanced hygiene practices in healthcare settings
- Vaccination: Preventing infections reduces the need for antibiotics
Research and Development
- New antimicrobials: Novel classes of antibiotics to overcome existing resistance mechanisms
- Alternative approaches: Bacteriophages, antimicrobial peptides, antibodies, and antivirulence compounds
- Diagnostics innovation: Point-of-care tests to guide appropriate prescribing
- Combination therapies: Using multiple drugs together to overcome resistance
Agricultural Reform
- Eliminating growth promotion: Phasing out antibiotic use for growth promotion in animals
- Improved animal husbandry: Better conditions reducing the need for preventive antibiotics
- Alternatives to antibiotics: Vaccines, probiotics, and improved biosecurity in animal production
- Surveillance: Monitoring antibiotic use and resistance in agriculture
Current Treatment Approaches for Resistant Infections
When faced with antibiotic-resistant infections, healthcare providers may employ these strategies:
Medical Interventions
- Combination therapy: Using multiple antibiotics simultaneously to overcome resistance mechanisms
- Higher doses or extended durations: Modified regimens that may overcome some forms of resistance
- Reserved antibiotics: Using “last-resort” medications typically held in reserve
- Susceptibility testing: Laboratory analysis to identify which antibiotics remain effective
- Source control: Surgical drainage or removal of infected material when possible
Emerging Therapeutic Options
- Bacteriophage therapy: Using viruses that specifically target bacteria
- Monoclonal antibodies: Targeting specific bacterial components
- Antimicrobial peptides: Naturally occurring molecules that disrupt bacterial membranes
- CRISPR antimicrobials: Gene-editing technology to target specific resistance genes
- Antivirulence approaches: Disarming bacteria without killing them, potentially reducing selective pressure
It’s important to note that treatment options vary widely based on the specific pathogen and its resistance pattern, emphasizing the need for proper diagnostic testing.
Prevention Strategies: Individual and Community Actions
While systemic changes are essential, individuals can also help combat antimicrobial resistance:
Personal Prevention Measures
- Use antibiotics only when prescribed: Never demand antibiotics for viral infections
- Complete the full course: Even if you feel better before finishing prescribed antibiotics
- Never share or use leftover antibiotics: Each prescription is specific to the person and infection
- Prevent infections: Regular handwashing, staying up-to-date on vaccinations, and safe food handling
- Report side effects: Informing healthcare providers about adverse reactions to antibiotics
Community-Level Prevention
- Awareness campaigns: Educational initiatives about appropriate antibiotic use
- Vaccination programs: Community-wide immunization reducing infection rates
- Safe disposal of medications: Proper disposal preventing environmental contamination
- Supporting antibiotic stewardship: Patient advocacy for appropriate prescribing practices
Home Management: Supporting Treatment of Infections
While home remedies cannot replace medical treatment for serious bacterial infections, these approaches may complement prescribed treatments:
Supportive Care
- Adequate rest: Allowing the body to direct energy toward fighting infection
- Hydration: Maintaining fluid intake to support immune function
- Nutrition: Consuming balanced diets rich in immune-supporting nutrients
- Fever management: Using appropriate over-the-counter medications as directed
- Symptom relief: Addressing specific symptoms like sore throat or congestion with appropriate remedies
Complementary Approaches
- Honey for coughs: Evidence supports honey as effective for cough suppression (not for children under 1 year)
- Salt water gargling: May help with throat discomfort from respiratory infections
- Steam inhalation: Can provide relief from nasal congestion
- Probiotics: May help restore gut microbiota during or after antibiotic treatment
- Rest and fluids: Supporting the body’s natural recovery processes
Important note: These approaches should supplement—never replace—medical care for bacterial infections. Always consult healthcare providers before trying complementary approaches.
The Future Outlook: Innovations in the Fight Against AMR
Despite the challenges, promising developments offer hope for addressing antimicrobial resistance:
Novel Discovery Approaches
- Artificial intelligence: Machine learning algorithms identifying new antibiotic candidates
- Mining unculturable bacteria: Exploring previously inaccessible microbial diversity for novel compounds
- Synthetic biology: Engineering microorganisms to produce new antimicrobial molecules
- Nanotechnology: Developing nanoparticle delivery systems to enhance antibiotic efficacy
Diagnostic Innovations
- Rapid molecular testing: Methods detecting resistance genes in hours rather than days
- Host biomarkers: Distinguishing between viral and bacterial infections to guide treatment decisions
- Point-of-care devices: Bringing sophisticated testing capabilities to frontline healthcare settings
- Surveillance technologies: Real-time monitoring of resistance patterns across populations
Economic Incentives
- Push incentives: Grants and public funding supporting early-stage research
- Pull mechanisms: Market guarantees and premium pricing for successful development
- Delinkage models: Separating company revenues from sales volume to encourage conservation
- Public-private partnerships: Collaborative approaches sharing risks and rewards of development
Global Collaboration
- One Health approach: Coordinated action across human, animal, and environmental health sectors
- Knowledge sharing: Open-access research and surveillance data
- Technology transfer: Ensuring innovations reach low- and middle-income countries
- Capacity building: Strengthening laboratory and regulatory systems globally
The Role of Public Awareness and Education
Combating antimicrobial resistance requires an informed public:
Key Educational Priorities
- Understanding when antibiotics are needed: Distinguishing between bacterial and viral infections
- Recognizing appropriate use: Following prescription instructions precisely
- Appreciating consequences: Understanding the individual and societal impacts of resistance
- Supporting policy change: Public backing for regulations on antibiotic use in agriculture and healthcare
Effective Communication Strategies
- Healthcare provider conversations: Clear explanations when antibiotics aren’t prescribed
- School-based education: Incorporating AMR awareness into science curricula
- Media campaigns: Evidence-based messaging through traditional and social media
- Community engagement: Local initiatives tailored to specific cultural contexts
Conclusion: The Path Forward in Combating Antimicrobial Resistance
Antimicrobial resistance represents one of the most significant public health challenges of our time—a slow-moving pandemic with potentially catastrophic consequences. Yet, unlike many global threats, the path forward is relatively clear, if challenging to implement.
Success requires unprecedented cooperation across sectors, disciplines, and national boundaries. Healthcare providers must embrace stewardship principles. Pharmaceutical companies need new incentives for antibiotic development. Agricultural practices must evolve. Policymakers should prioritize long-term public health over short-term interests. And individuals must become responsible antibiotic users and advocates.
The stakes could not be higher. Without effective antibiotics, modern medicine as we know it—from routine surgeries to cancer treatments—becomes enormously more risky. Common infections could once again become life-threatening. But with coordinated action, we can preserve the miracle of antimicrobials for future generations.
As the WHO emphasizes: “No action today, no cure tomorrow.” The time for that action is now.
Frequently Asked Questions About Antimicrobial Resistance

Q: Is antibiotic resistance the same as antimicrobial resistance?
No. Antibiotic resistance specifically refers to bacteria becoming resistant to antibiotics. Antimicrobial resistance is broader, encompassing resistance development in all microorganisms (bacteria, viruses, fungi, and parasites) against respective drugs.
Q: Can bacteria lose their resistance to antibiotics?
Yes, but rarely. Maintaining resistance mechanisms often comes with energy costs for bacteria. Without continued antibiotic pressure, some resistant strains may be outcompeted by non-resistant strains over time. However, this process is typically much slower than resistance development.
Q: Does antibiotic resistance affect everyone equally?
No. Those who are immunocompromised, chronically ill, very young or elderly, or require frequent medical care face higher risks from resistant infections. Geographically, resistance rates vary widely, with some regions facing far higher prevalence of resistant organisms.
Q: antibiotics in food cause resistance in humans?
Potentially. While the relationship is complex, evidence suggests resistant bacteria can transfer from animals to humans through the food chain. Additionally, antibiotic residues in food may impact human gut microbiota, potentially contributing to resistance development.
Q: Is antimicrobial resistance a natural process?
Yes, resistance is a natural evolutionary process, but human activities—especially antibiotic misuse—have dramatically accelerated it. Bacteria have existed for billions of years and have naturally evolved defense mechanisms against threats, including antimicrobial compounds.
Q: Are pharmaceutical companies developing new antibiotics?
Unfortunately, investment in new antibiotic development has declined significantly. The current business model makes antibiotics less profitable than drugs for chronic conditions. However, various initiatives, including public-private partnerships, are working to revitalize the antibiotic pipeline.
Q: Can I do anything to fight antimicrobial resistance?
Absolutely. Use antibiotics only when prescribed and exactly as directed, practice good hygiene to prevent infections, stay up-to-date on vaccinations, and choose animal products from producers who use antibiotics responsibly. Additionally, raise awareness among friends and family about this critical issue.Q: Is antimicrobial resistance reversible?
At the population level, resistance can potentially be reduced through stringent antimicrobial stewardship and infection control measures. However, once resistance genes become widespread, completely reversing resistance becomes extremely challenging—emphasizing the importance of prevention.
ALSO READ MORE HEALTH ARTICLES FROM CHIID HEALTH